Virtually every case of Covid-19 - no matter how severe, no matter how upsetting to the person it affects - begins with something mundane. A handshake. A conversation. Or maybe a cough.
A few thousand droplets are flung into the air with each cough by someone suffering from a respiratory illness. But that is only the vanguard, the heavy cavalry: These droplets, a few thousandths of a millimeter in size, giants in the world of viruses, fall to the ground after a few arm's lengths at the latest.
More numerous and more insidious are the aerosols: particles so small that they can remain suspended in the air for long periods. A few minutes, sometimes even more than twelve hours. Hundreds of thousands of aerosol particles can be catapulted into the air by a single sneeze or cough, and of course they are also produced when we sing or speak.
And if the other person is unlucky, some of these airborne particles contain an even tinier thing: Sars-CoV-2, also known as the coronavirus.
As of now, it is considered certain that the coronavirus spreads via the air in this way. But how does the virus get from this envelope into the lungs and cells of a new victim? And what happens inside the aerosol? It all happens so quickly and on such a small scale that it is impossible to observe - or is it not?
A team of around 50 researchers has now taken a major step forward in deciphering the behavior of the coronavirus at the atomic level - and has discovered some of the features that are believed to make the virus so successful. The scientists led by biophysicist Rommie Amaro of the University of California San Diego (UCSD) have simulated a complete aerosol particle on a supercomputer for the first time, with everything inside.
These are not just pretty illustrations of the nanoworld, dreamed up by an artist. The scientists have built the model atom by atom, with all the information known about the structure of Sars-CoV-2. 305 million atoms comprise the model of the virus alone. The whole aerosol contains more than a billion atoms. Calculating it required the "Summit" at the U.S. Oak Ridge National Laboratory, the second most powerful supercomputer in the world. "This is pretty much the largest model ever built for a biological simulation," says Rommie Amaro, head of the San Diego-based group. Her full job title is computational biophysical chemist, which also gives an impression of the wide range of skills needed: a mix of computer science, biology, physics and chemistry. In total, over a dozen universities and research institutions were involved in the work.
Typically, different research groups look at individual components of the virus, Amaro says. Some observe only the spike protein under a microscope, while others study the behavior of the membrane or of the aerosol. "The cool thing about our approach is: we can combine these different experimental data to create these highly detailed 3-D models." In this way, the individual pieces of the puzzle come together to form a complete picture.
But the real strength of such "molecular dynamics simulations" (MD) lies not in individual images, but in the fact that they can show a virus in action, as in a movie. Only then is it possible to see how the individual components interact - and which ones the pathogen might benefit from.
This leads the researchers to assume that the mucins shield the Spike proteins within the aerosol, for example from harmful influences such as sunlight. This protective layer of mucins could explain why Sars-CoV-2 spreads so successfully through the air.
In this way, the simulations can lead to insights about the virus. "If we see something like we did with the mucins, we can use that to think of new experiments," Amaro says. Other researchers could then test those hypotheses in laboratory experiments.
For the predictions to be reliable, the researchers must calculate what attractive and repulsive forces each atom exerts on every other atom - this is the only way to determine the motion of all the particles. Helmut Grubmüller of the Max Planck Institute for Multidisciplinary Natural Sciences in Göttingen compares the effort involved with the "party handshake problem": How many times do guests at a party have to shake hands in total so that everyone has greeted everyone else? With five guests it would be ten handshakes, with 25 people it would be 300. And with 300 million atoms, as in the case of the simulated virus particle? You end up with 44 million billion, a number with 17 digits.
At the same time, the forces are constantly changing, because the atoms are in motion. "This means that you have to calculate all the forces again very shortly afterwards," says Grubmüller. As head of the Theoretical and Computational Biophysics research group in Göttingen, he simulates proteins using methods similar to those used by Amaro. Repeat the calculation a few million times, and you may have captured a few millionths of a second in the existence of a virus or protein. However, Grubmüller stresses, "these are approximations. You can't believe this blindly." It is extremely important, he says, to keep checking whether what you calculate is also confirmed experimentally.
The biophysicists are aided by the fact that microscopy has also advanced into ever smaller dimensions in recent years. Thanks to techniques such as cryoelectron microscopy (cryo-EM), viruses can be photographed with atomic precision. This structural elucidation also provides input for the simulations, but cannot replace them. Grubmüller illustrates this with the example of an internal combustion engine: a photo of it would show the pistons and crankshafts, but not how they rise, fall or rotate - and with a long-term exposure everything is blurred anyway. "Most proteins also function by moving," Grubmüller says.
But it's not just in the aerosol around the virus that particles buzz around, according to the U.S. simulations - the virus itself also moves.
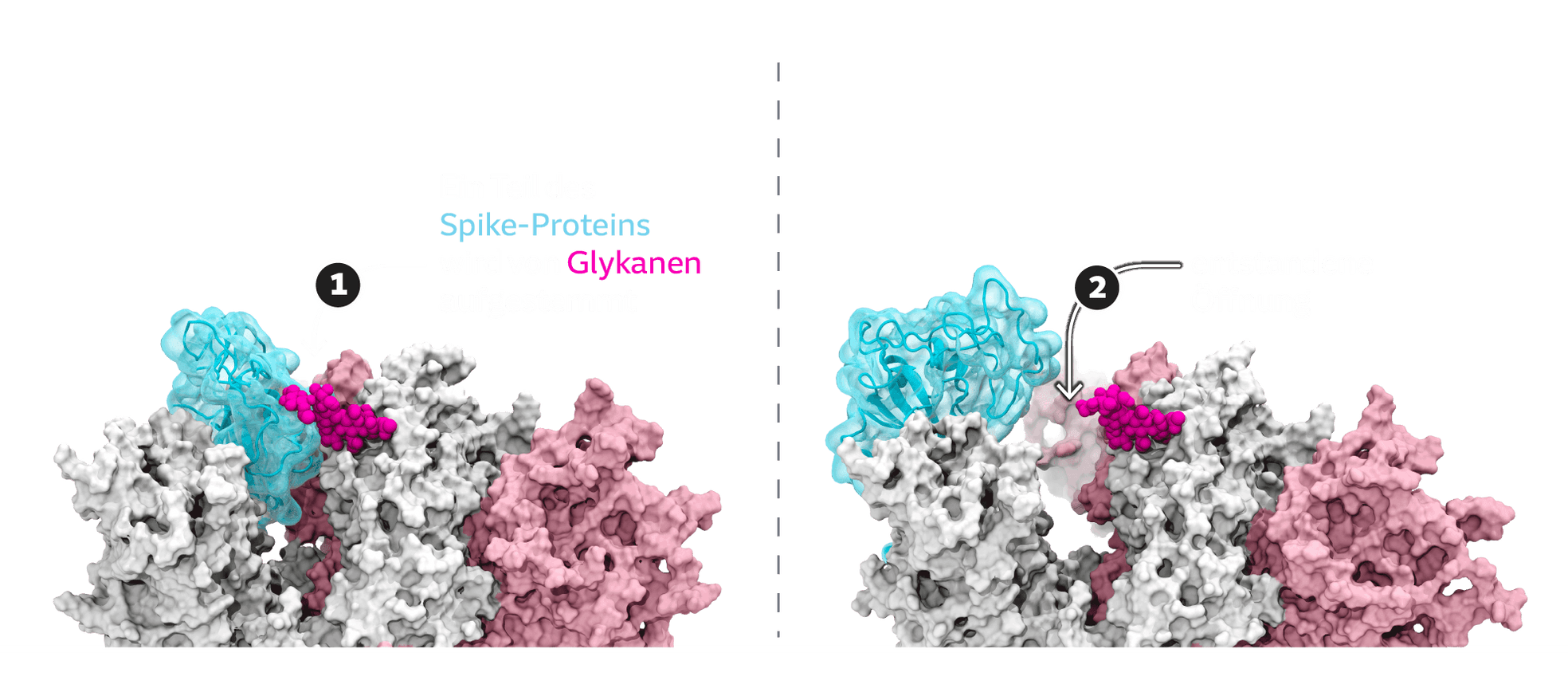
As the calculations show, the tip of the Spike protein can switch between different positions. Most of the time, it is closed. "To infect a cell, the spike protein must open," Amaro says. That's because this opening dramatically increases the affinity for the docking site. In a sense, the Spike protein switches into an attack formation, like a snake opening its mouth just before it bites:
Glycans, long-chain sugar molecules, are also deposited on the spines. Thanks to this sugar coating, the virus presumably slips through the ranks of the body's immune defenses quite easily. After all, it's only sugar, what harm could it do? The calculations show that the glycans are also involved in opening the sting: Like a chisel, they pry open the protein.
However, not every variant is equally dangerous: it is known, for example, that the Delta variant binds significantly better to the ACE2 receptor of human cells than the original Sars-CoV-2. The simulations have helped to show why: Delta's sting opens significantly wider than the original protein - the mouth snaps open further, if you like.
The rapid adaptation to new variants is a particular strength of the approach: as soon as the mutations in the spike protein are known, they can in principle be calculated. However, this is only possible up to a certain point. The Omicron variant, for example, has so many altered sites in the Spike protein alone that they could affect the behavior of the entire construct. One must therefore be cautious with predictions, says Amaro.
But also the antidotes. With the help of supercomputers, it is possible not only to screen the virus, but also to design countermeasures. "In the future, the simulations will become very important in drug development," predicts Grubmüller. They can show, for example, how tightly a potential drug binds to a target protein such as the Spike, allowing conclusions about its effectiveness. In this way, thousands of possible components can first be tested in the simulation before moving on to laboratory trials. "This allows you to see where there is room for improvement and helps to design a drug in a much more targeted way."
A research team led by Yale University chemist William Jorgensen has already used this approach during the pandemic. The scientists virtually pitted drugs approved for other diseases against an enzyme in Sars-CoV-2 that controls viral replication. This way they initially reduced the selection to 14 candidates, and are now further optimizing some that are promising.
"These images are so challenging that we have melted some motherboards"
Amaro's group is currently working on a complete model of the Omicron variant. The researchers are also benefitting from advances in graphics cards, which were originally developed for video games. They have probably multiplied the speed of simulations in recent years.
Nevertheless, the available computing power has so far imposed tight limits on the researchers. In all, the researchers used the Summit computer to simulate 2.42 nanoseconds (billionths of a second) in the existence of the aerosol. "These images are so challenging that we melted some motherboards," Amaro says. "I was laughing and crying at the same time." In five to ten years, Grubmüller estimates, "we could be at the level where the aerospace industry was ten years ago." Today, aircraft have long been tested in a virtual wind tunnel before the first prototype is bolted together.
Her dream is to eventually be able to simulate the complete infection pathway, Amaro says: from the lungs into the aerosol, through the air into the next lung, the next cell. "That would be the next big step. But we need bigger computers to do that."